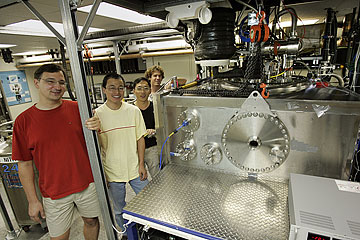
FL MORRIS / FMORRIS@STARBULLETIN.COM
University of Hawaii researchers Ralf Kaiser, left, Fangtong Zhang, Xibin Gu and Corey Jamieson yesterday stood next to the "Crossed Molecular Beams Machine," which simulates the atmosphere of the moon Titan. CLICK FOR LARGE
|
|
UH-led researchers to study chemistry of Saturn's moon
Scientists hope they will get clues as to how the Earth's own atmosphere formed
A University of Hawaii physical chemist is leading an international group of researchers in studies of Saturn's moon Titan, which may reveal how the Earth began billions of years ago.
"Many people think Titan and our Earth were formed initially with similar compositions," Ralf Kaiser, associate professor at UH-Manoa, said yesterday.
"If you understand the chemistry of Titan, you can look back in time to see the chemistry of how our own Earth developed."
Kaiser recently received a five-year, $2.4 million grant from the National Science Foundation's Collaborative Research in Chemistry Program for his interdisciplinary group's Titan work. It was one of six grants awarded out of 118 proposals submitted.
Kaiser's team built a special machine for the research after he joined UH four years ago. They obtained the first results about 18 months ago, he said.
"Since we can't go to Titan, which is too far out, we have to do experiments in the laboratory. It mimics or simulates the chemistry in Titan's atmosphere," he said.
There are only two other machines similar to his in the United States and a few in Europe and Taiwan, Kaiser said. "It's a pretty complicated system," he said. It's called the "Crossed Molecular Beam Machine."
He said Titan's chemistry is known from recent space missions.
"It contains very complicated hydrocarbon molecules. We can simulate these and more complicated molecules that are formed," he said.
Titan has a relatively low temperature, about minus 290 degrees Fahrenheit, with lots of chemistry, he said. "It's more or less like a refrigerator, with no liquid water."
The major objective is to understand the formation and growth mechanisms of the molecules and apply the findings to better understand Titan's hydrocarbon chemistry, Kaiser said.
Out of that may come clues enabling scientists to recreate the Earth's atmosphere in its infancy, he said, explaining primordial Earth's atmosphere and Titan's atmosphere are believed to have formed with similar chemistry from the Solar Nebula.
Hydrocarbon molecules in Titan's atmosphere also absorb the sun's ultraviolet radiation, creating a relatively low surface temperature that acts as "prebiotic ozone" to preserve astrobiologically important molecules, Kaiser said.
Based on their experiments, the researchers can predict which molecules are in Titan's atmosphere and Alan Tokunaga, with the UH Institute for Astronomy, can look for them in the infrared spectra, Kaiser said.
He said a "tightly integrative collaborative network" will be developed with comprehensive studies in electronic structure theory, photochemistry, reaction dynamics and kinetics, as well as applications in planetary chemistry and observations of Titan with the infrared telescope.
Observational and laboratory results will go to Yuk Yung, California Institute of Technology planetary scientist, who will feed them into models to "go back into the past and also the future," Kaiser said.
Other mainland collaborators are calculating the chemical reactions, he said.
The UH group's closest competitors in understanding Titan's atmosphere are in Italy, but they're investigating different reactions, Kaiser said.
"In Titan's atmosphere, you have different chemicals. It's impossible to study all the different reactions at one time. They're completely different systems than we're studying."

